- What is TOPOGRAPH?
-
TOPOGRAPH (Therapy-Oriented Precision Oncology Guidelines for Recommending Anti-cancer PHarmaceuticals) is an independent, oncologist-led effort of cataloguing literature and evidence to streamline the process of recommending drug treatments in precision cancer medicine.
This resource is intended to help oncologists find relevant literature when making treatment decisions for patients with advanced or metastatic cancer, using contemporary genomic profiling results and other assays.
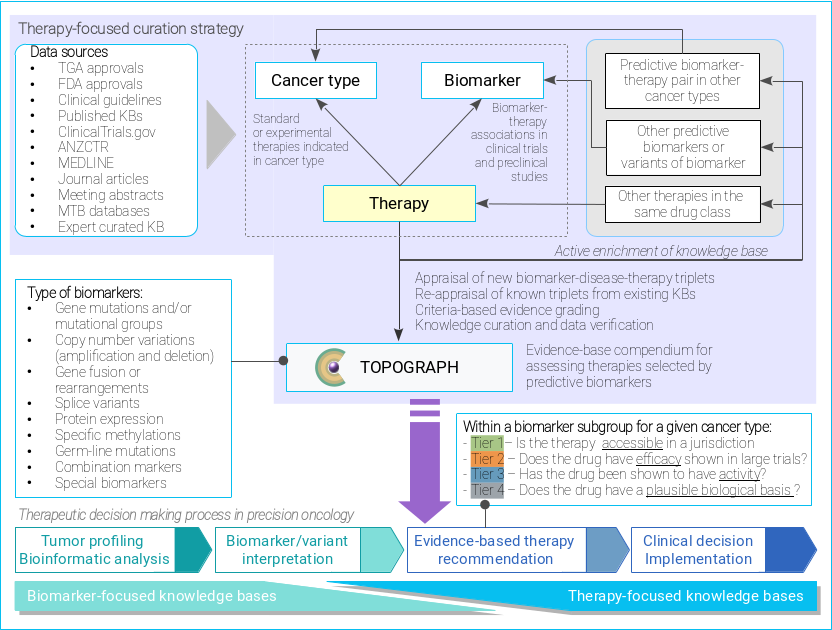
TOPOGRAPH is a knowledgebase of predictive biomarkers in oncology and haematology, linking their therapeutic effects of anticancer drugs on the basis of published evidence. The types of biomarkers linked to therapies include but are not limited to:
- Gene biomarkers, including:
- Simple mutations and variant groups
- Copy number variations (amplification and deletions)
- Fusions and rearrangements
- Germline mutations
- Combination biomarkers
- Complex biomarkers
- Splice variants
- Protein biomarkers by expression categories
- Selected methylations
- Selected genome-wide assays and/or metrics
- Selected predictive scores
Currently, TOPOGRAPH provides contents with reference to the Australian public health system. This resource is actively maintained as part of a nation-wide precision oncology program.
- Gene biomarkers, including:
- Why do we need another knowledge base?
-
There are a number of precision oncology knowledgebases (KBs) available, such as
OncoKB
, Cancer genome interpreter
, PMKB
, JAX CKB
, CIViC
, and ClinVar.
Efforts in harmonisation of different KBs for variant interpretation have also been established.
Most other publicly available KBs focus on delineating the significance of genomic alterations found through a diagnostic assay (e.g., comprehensive genomic profiling).
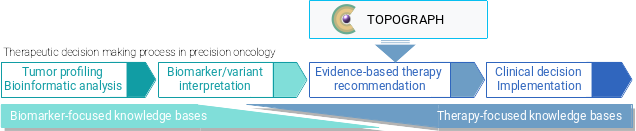
In contrast, TOPOGRAPH addresses the real-world need of oncologists by focusing on the treatment selection aspects (hence "therapy-oriented").
TOPOGRAPH uses literature and evidence to understand the implications on a treatment (drug or drug combination) in the presence of a biomarker, identified by methods of molecular profiling and/or other assays (tests).
TOPOGRAPH is designed differently from other databases.
To make this resource useful in clinics, TOPOGRAPH considers both accessibility (with respect to approval and reimbursement) and maturity of a drug (with respect to its stage of development, as well as the outcomes of relevant clinical trials) more closely.
These aspects are important to consider when making rational clinical decisions about targeted cancer therapies.
More general discussions about the rationale behind this resource can be found here .
- Why are AMP/ASCO/CAP or ESCAT criteria not used for cataloguing the therapies?
- The AMP/ASCO/CAP criteria (Li et al. J Mol Diagn 2017) was purposed to annotate clinically relevant variants from diagnostic molecular profiling. The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) (Mateo et al. Ann Oncol. 2018) criteria was designed for ranking genomic alterations as targets in precision oncology. While both AMP/ASCO/CAP and ESCAT criteria provide valuable frameworks for evidence assessment, neither criteria can represent drug resistance/lack of drug activity succinctly, which is also important in clinical decision-making. TOPOGRAPH adapts a grading/numbering system similar to OncoKB but defines a different set of literature criterias to align more closely with the needs of oncologists in practice (See Literature assessment criteria).
- Can TOPOGRAPH be used for assessing the oncogenicity of a variant?
- TOPOGRAPH is not intended for interpreting the pathogenicity of gene variants in cancer. Expertise in molecular pathology and other resources should be consulted for this task, in addition to bioinformatic analysis, before TOPOGRAPH is used to assess whether a therapy is relevant in the presence of a variant or a biomarker. Established therapeutic guidelines should always be consulted.
- What is the latest database version?
- The latest version of the database is 20251215 (AU). See Change log.
- Can I refer a patient for genomic profiling?
- TOPOGRAPH provides information about targeted therapies and relevant biomarkers only and cannot accept referrals for genomic profiling.
For access to genomic profiling options for Australian patients, please consider referral to:
- The Cancer Screening Program (CaSP) (provided by Omico Ltd.) and its Molecular Oncology Board.
- An accredited laboratory with clinical comprehensive genomic profiling services.
- Referral to dedicated research programs may be appropriate for certain patients.
- Can I seek advice regarding patient treatment or the interpretation of genomic profiling results?
- TOPOGRAPH is a knowledge source that provides information about targeted therapies and relevant biomarkers only and does not provide clinical service.
For interpretation of genomic profiling findings, patients should always consult their primary oncologist for interpretation of results and participation in biomarker-matched clinical trials when clinically appropriate.
Specialist consultation of genomic profiling findings is always essential in cancer care. Case review through a Molecular Tumour Board (MTB) or a specialised Molecular Multidisciplinary Team (MDT) is considered best practice for patients with cancer, in alignment with international guidelines.Quaternary referral services for treating oncologists are available for Australian patients with cancer, in partnership with publicly funded health services research:
- The Precision Care Clinic at Prince of Wales Hospital, Sydney, provides a service that partners with clinicians to navigate complex molecular findings through a formal MDT input after genomic profiling. The team combines expertise from medical oncology, clinical genetics and molecular pathology to enhance genomic result interpretation within each patient's unique clinical context. Face-to-face or telehealth reviews are available for patients when required. This service is part of a national health services research initiative, funded by Australia's Medical Research Future Fund (MRFF Rapid Applied Research Translational grant, RARUR000125), which aims to develop sustainable models in the Australian public health system for incorporating genomics into routine cancer care. To refer a patient, please use the online link: Referring clinicians - Precision Care Initiative | UNSW Sydney. There are no costs to patients or referring clinicians.
- Is there an analytic tool or a clinical trial matching function?
- A suite of online tools is being developed for matching patients to Australian find cancer trials based on results of comprehensive genomic profiling. For information about cancer clinical trials, ARTICANZ - Annotated Registry of Trials in Cancer (Australia and New Zealand Ed) (Annotated Registry of Trials in Cancer, Australia/NZ Edition; ARTificial Intelligence-enhanced CANcer trial information database) is a free, open-information initiate designed to provide comprehensive categorisation of cancer drug trial information to oncologists/haematologists, researchers, trialists, patients, and different stakeholders in Australia and New Zealand.
- Is TOPOGRAPH computable?
-
Yes. TOPOGRAPH can be computationally accessed through the POTTR (Precision Oncology Treatment and Trial Recommender) tool, an open-source, Perl-based clinical decision support system available on GitHub .
POTTR is designed to integrate patient genomic testing results with TOPOGRAPH's knowledge base to produce evidence-based treatment recommendations. The system can:
- Rank potential therapies based on strength-of-evidence and level-of-recommendation, based on TOPOGRAPH
- Predict drug sensitivity and resistance by analyzing variant interactions
- Match patients to relevant clinical trials using local trial databases
- How do I cite TOPOGRAPH in a scientific paper?
- Lin FP, Thavaneswaran S, Grady JP, Ballinger M, Kansara M, Oakes SR, Desai J, Lee CK, Simes J, and Thomas DM. Criteria-based curation of a therapy-focused compendium to support treatment recommendations in precision oncology. npj Precis Oncol 2021; 5: 58. doi: 10.1038/s41698-021-00194-z
When using data from TOPOGRAPH, please:
- State the database version accessed and date of access
- Acknowledge the appropriate licensing terms (see Licensing & Usage tab)
- If you modify or build upon TOPOGRAPH data, ensure your derivative work is also licensed under CC BY-SA 4.0
- How do I report errors or make suggestions about the website/database?
- Please contact Frank Lin for website issues and corrections of curated contents.
Licensing Overview
TOPOGRAPH is available under a dual licensing model designed to encourage both academic research and commercial innovation while protecting the integrity of the database and ensuring improvements benefit the precision oncology community.
Database Usage License
For incorporating, displaying, or using TOPOGRAPH data✅ Permitted Uses:
- • Displaying data in clinical software interfaces
- • Querying the database to provide treatment recommendations
- • Incorporating data into clinical decision support systems
- • Creating visualizations or reports based on TOPOGRAPH data
- • Building APIs that serve TOPOGRAPH data
- • Training machine learning models on TOPOGRAPH data
- • Commercial products using TOPOGRAPH data
📋 Requirements:
- • Attribution: Credit TOPOGRAPH and provide a link to this resource
- • Version citation: State database version and access date
🚫 No Requirement to:
- • Make your software open source
- • Share proprietary algorithms or code
- • Apply ShareAlike to your entire product
Database Modification License
CC BY-SA 4.0 for modifying or augmenting the database🔧 What Triggers ShareAlike:
- • Adding new drug-biomarker associations
- • Modifying the tier classification system
- • Augmenting entries with additional data fields
- • Creating translated or region-specific versions
- • Merging TOPOGRAPH with other databases
- • Redistributing modified database files
📋 Requirements:
- • Attribution: Credit TOPOGRAPH and indicate changes made
- • ShareAlike: Modified versions must use CC BY-SA 4.0 license
- • Community Benefit: Improvements should benefit the precision oncology community
Key Distinctions
Why ShareAlike Licensing?
TOPOGRAPH adopts a ShareAlike approach for database modifications to foster collaborative knowledge sharing in precision oncology. This ensures that improvements to the core database benefit the entire precision oncology community.
Our Philosophy:
- Open Science: Accelerate discovery through transparent, collaborative research
- Clinical Impact: Ensure advances in biomarker-informed therapy reach all patients
- Community Benefit: Knowledge improvements should enhance precision oncology for everyone
- Sustainable Innovation: Balance commercial development with public good
By requiring that database modifications remain open, we create a sustainable ecosystem where commercial innovation thrives while core knowledge remains accessible to researchers, clinicians, and patients worldwide. This approach has proven successful in other scientific domains and aligns with best practices in precision medicine data sharing.
Commercial Use
Yes. Commercial products that use TOPOGRAPH data are explicitly encouraged. You can build clinical decision support software, integrate TOPOGRAPH into proprietary healthcare platforms, create subscription services, or develop mobile applications using this data.
We believe this approach encourages innovation in precision oncology tools while ensuring improvements to the core database benefit everyone.
Contributing Improvements
We welcome contributions! If you've identified errors, have additional literature evidence, or want to suggest new therapeutic associations, please contact Frank Lin to discuss your contributions.
TOPOGRAPH does not accept responsibility for the accuracy or completeness of the curation content.




