- Tier 1 – Standard-of-care therapy using the biomarker for therapy selection and approved by TGA
- T1 Publicly funded medicine TGA-approved therapy indicated by the presence of biomarker, and reimbursed by PBS
- T1B Regulatory approval TGA-approved therapy indicated by the presence of biomarker, but not reimbursed by PBS
- Tier 2 – Standard-of-care therapy using the biomarker for therapy selection, but not approved by TGA
- T2 Regulatory approval Therapy indicated by this biomarker, not approved by TGA, but approved by other international jurisdiction(s), e.g., FDA or EMA, in the indication.
- T2 Clinical guidelines Therapy indicated by this biomarker, as endorsed by established clinical guidelines e.g., eviQ (Australia) or NCCN (Category 1 or Category 2A with strong supporting literature).
- T2 Phase 3 clinical trials Therapy with positive efficacy results in ≥1 studies with prospective biomarker selection.
- T2 Phase 2 clinical trials Therapy with exceptional efficacy results in ≥1 studies with prospective biomarker selection.
- T2 Phase 2 basket trials Therapy with exceptional efficacy results in ≥1 studies with prospective histotype subgroups.
- Tier 3 – Therapy with strong clinical evidence of anti-tumour activity in the presence of the biomarker.
- T3 Phase 3 clinical trials Therapy with positive anti-tumour activity demonstrated in a retrospective or exploratory biomarker subgroup analysis in a Ph3 study; inconclusive or conflicting subgroup efficacy data in Phase 3 trials.
- T3 Phase 2 clinical trials Therapy with positive anti-tumour activity demonstrated in ≥1 prospectively and biomarker-selected Phase 2 trials, defined as meeting its primary endpoint and included in preplanned biomarker subgroups
- T3 Phase 2 basket trials Therapy with positive anti-tumour activity demonstrated in ≥1 prospectively and positive prospective Phase 2, including pre-planned histotype-specific subgroups
- T3 Phase 1 clinical trials Therapy with positive anti-tumour activity demonstrated in ≥1 well-sized, prospectively biomarker-selected Phase 1 studies that demonstrates exceptional activity
- Tier 4 – Therapy with strong preclinical or early clinical evidence of anti-tumour activity in the presence of the biomarker
- T4 Phase 2 clinical trials (including basket trials) Therapy showing probable anti-tumour activity in an exploratory biomarker subgroup in phase 2 clinical trial(s).
- T4 Phase 1 clinical trials Therapy showing probable anti-tumour activity in dose expansion phase of a Ph1 clinical trial with prospectively defined biomarker subgroups
- T4 Retrospective studies Real-world data registry Systematic reviews Therapy showing probable anti-tumour activity identified from a well-sized patient registry, retrospective cohort study, or literature reviews.
- T4 Case report case series Therapy showing probable anti-tumour activity as identified by efficacy or objective response in ≥1 case reports or series, regardless of prospective or retrospective selection by biomarker.
- T4 Clinical trial registries Therapy studied in a clinical trial where the biomarker is selected as an inclusion criteria (any phase).
- T4 Pre-clinical research Therapy with strong preclinical rationale cell-line or animal studies suggestive of anti-tumour activities.
- Tier R1 – Standard-of-care therapy not recommended in the presence of the biomarker, as listed in regulatory approval documents or clinical guideline
- R1 Regulatory approval Therapy where the biomarker is explicitly listed as a contraindication (to a therapy that may otherwise be prescribed for the indication) in regulatory approval documents, suggesting a lack of efficacy in this biomarker subgroup.
- R1 Clinical guidelines Therapy where the biomarker is explicitly listed as a contraindication (to a therapy that may otherwise be prescribed for the indication) in an established clinical guideline, suggesting a lack of efficacy in this biomarker subgroup.
- Tier R2 – Therapy predictive of lack of anti-tumour activity in the presence of the biomarker based on compelling clinical or preclinical evidence
- R2 Phase 2/3 clinical trials Therapy where the biomarker or histology subgroup (basket trial) in ≥1 clinical trials showed below expected response for anti-tumour activity
- R2 Retrospective studies Real-world data registry Systematic reviews Therapy where a well-sized biomarker subgroup is identified from the sources with a below expected response of anti-tumour activity
- R2 Case report/case series Therapy where published case reports or case series showing below-than-expected response of anti-tumour activity
- R2 Clinical trial registries Therapy examined in a clinical trial (any phase) where the biomarker is listed as an exclusion criteria suggestive of resistance, lack of anti-tumour activities or efficacy.
- R2 Pre-clinical research Therapy examined in a well-structured drug sensitivity studies, or exploratory analysis of tumor progression, that suggest a plausible mechanism where the biomarker is associated with lack of response to therapy, including both intrinsic and acquired mechanisms.
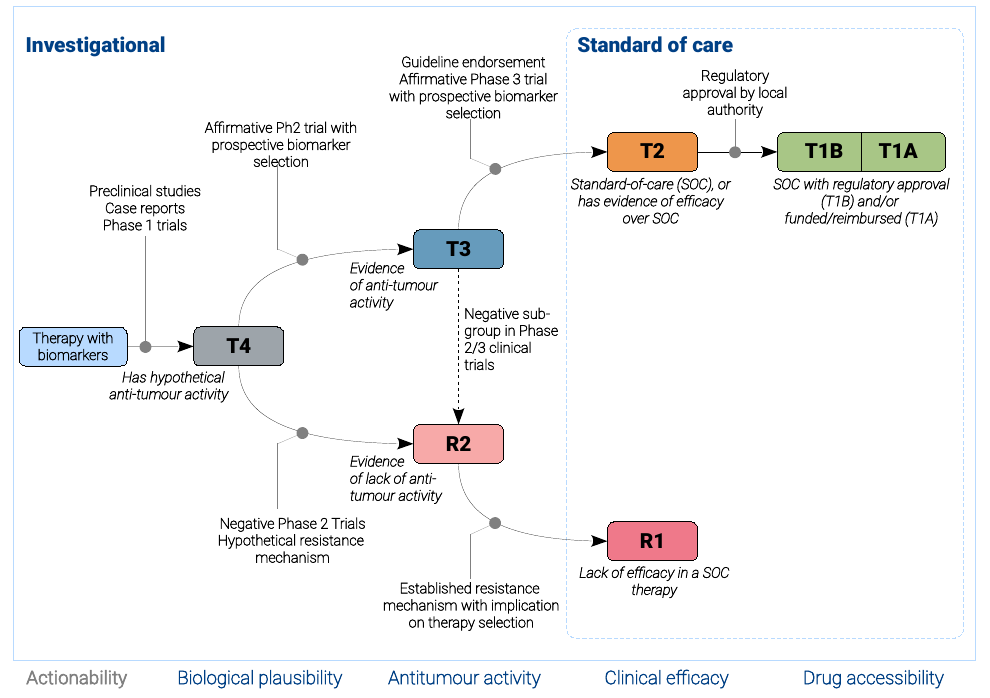
TOPOGRAPH defines evidence tiers in reflects maturity of drug development with respect to maturation in drug development progress.
T1, T2, and R1 are considered standard of care, whereas the remaining tiers are considered experimental.
Therapy yet to be adequately investigated for possible clinical activities are designated T4, considering that lacking evidence about
anti-tumour activity is not equivalent to evidence of lack of activity (R2).
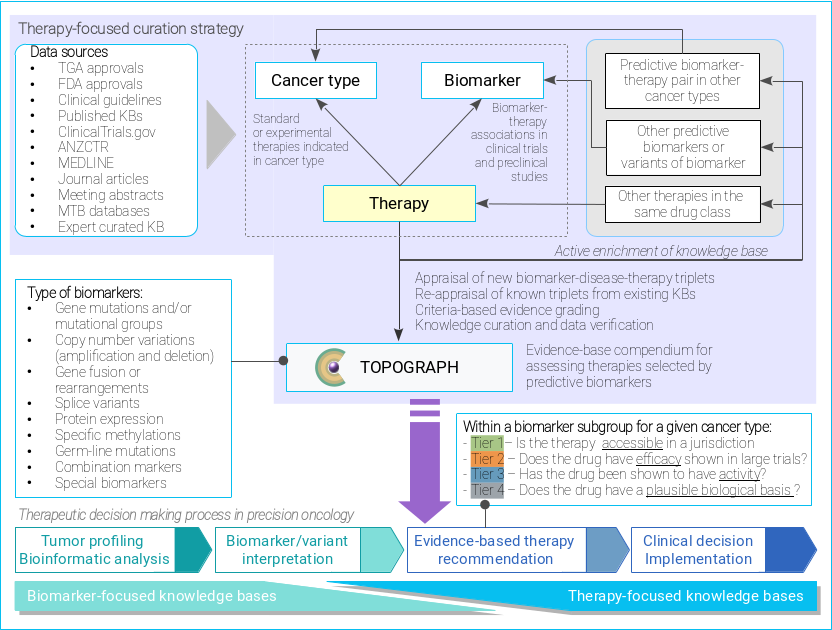
Curation strategy of TOPOGRAPH.
ANZCTR: Australia and New Zealand Clinical Trial Registry.
KB: knowledge base.
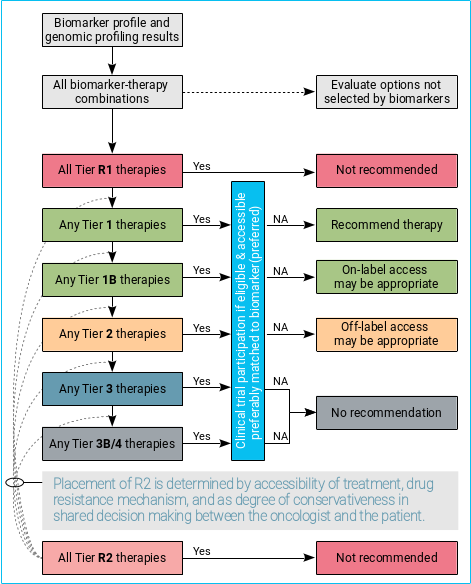
To make this resource more accessible in the clinic, a cascade decision algorithm is proposed to facilitate the discussion and shared decision-making about different options of targeted cancer treatments between a patient and his/her treating oncologist.
In general:
- Biomarker-driven therapies should always be considered alongside other therapeutic options not selected based on biomarker, and supportive care.
- Participation of clinical trial is always considered the best clinical practice, if one is accessible.
- T1 therapies are recommended with exception of when a concomitant, high-level resistance biomarker is present (i.e., Tier R1) or known failure of treatment after previously exposure, intolerance, or toxicity to another drug in the same therapeutic class.
- Off-label use of T2 therapies may be considered appropriate in selected circumstances.
- T3/4 therapies are not generally recommended outside clinical trial settings, given lack of compelling clinical data to support its use.
- In exceptional circumstances where treatment options are limited for a given cancer type, off-label access of therapies with lower tier (T3/4) may be considered appropriate, if the potential value of accessing a therapy outweighs the potential harms.




